
WWW
Goals of IA45
IA45 will establish UHN as a premier destination for clinical research through the goals listed below.
Speed
Ease
Collaboration
Transparency & Accountability
IA45 Timeline
Click each phase for more detail
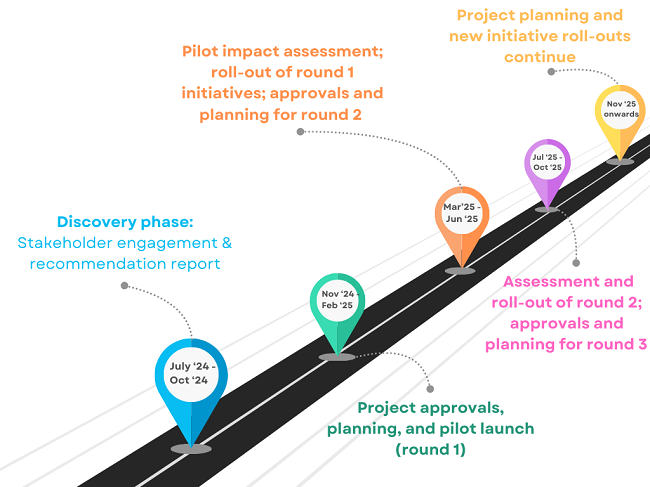
- Mapping of entire review and approval process
- Study Team survey
- First wave of IA45 initiatives pilot planning
- Launch pilot for first wave initiatives; Abbreviated IA Process, 14-day Objection Window, Fast-Track Stream
- Coming Soon
- Coming Soon
- Coming Soon
- Communication and Outreach
Check out the first Clinical Research Collaborative Centre Town Hall where several members of the clinical research executive presented updates, highlights and future plans for the Clinical Research Collaborative Centre.
For more information, check out this PDF and stay tuned for exciting updates coming your way soon!
- Initiatives UnderwayAbbreviated IA Process
Pilot an abbreviated IA process to remove non-mandatory reviewers. Separate approval streams, one focused on only mandatory approvals for IA and a second track for all the UHN business process reviews that can happen in parallel or after IA.
14-Day Objection Window
Movement towards attestation or opt-out models for review. This model would provide an implied approval within 28 days if no objection is made in the 1st 14 days from submission. This initiative aims at balancing the need for efficiency with regulatory compliance and aims to provide transparency and accountability to all stakeholders involved in the review process.

Fast-Track Stream
Creation of an IA45 study “fast-track” lane for agreed-upon studies, including standardizing expectations of stakeholders to meet fast-track timelines. This initiative aims at enabling Principal Investigators (PIs) to identify and recommend priority studies for endorsement, and providing clarity for review departments on where to focus their efforts.

- Initiatives Implemented
Clinical Trial Agreements (CTA) initiative completed: A master template agreement has been created through Clinical Trials Ontario (CTO) to use between UHN and participating sponsors in order to vastly reduce negotiation time for clinical trials.
- Accountabilities
The UHN Clinical Research Collaborative Centre is a strategic initiative since 2023 designed to centralize and enhance and enable clinical research across the institution. Through the establishment of Clinical Research Units (CRUs) at each hospital site, the Clinical Research Collaborative Centre provides essential infrastructure, streamlined support, and expertise to optimize the conduct of clinical trials.
The Clinical Research Collaborative Centre will play a pivotal role in Sponsorship and implementation of IA45 by leveraging the infrastructure and expertise of all activities involved in study approval to streamline research processes, ensure the consistent application of standardized guidelines, and support investigators in navigating the authorization workflow. This includes supporting Research Ethics Boards (REB) and contracts to ensure that IA45 goals are achieved while maintaining the highest standards of quality and integrity in clinical research. By fostering efficiency, mitigating risk, and promoting a culture of safety and quality, the Clincal Research Collaborative Centre strengthens UHN’s reputation as a global leader in clinical research, positioning the institution as a preferred partner for clinical research studies.
- FAQs
- How does UHN plan to support departments through this initiative?
UHN is committed to ensure successful implementation by providing comprehensive support at every stage. The intention is to enhance business process by reducing transactional activities, and ultimately reduce workload.
- What is Institutional Authorization?
Institutional Authorization is the process by which all human research studies are reviewed and approved at UHN. It helps research institutions minimize risk, identify opportunities for efficiencies, and promote a culture of safety and quality in clinical research.
- What is IA45?
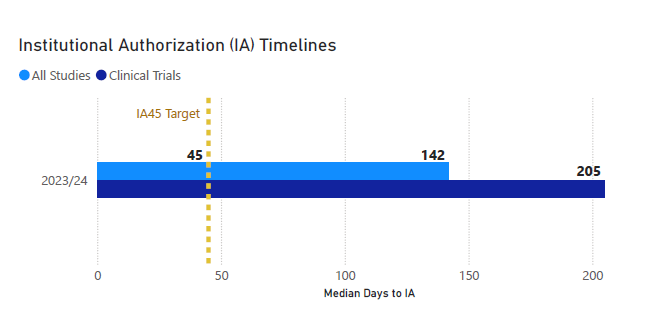
IA45 is a major transformation project undertaken by UHN to improve the Institutional Authorization process. The goal of IA45 is to reduce the average time for activating research studies to 45 days, with commitment from all stakeholders involved.
- Why is IA45 important?
IA45 aims to expedite the research study start-up process while maintaining the quality and integrity of clinical research. By doing so, UHN seeks to establish itself as a preferred partner for clinical research and enhance its impact within the industry.
- How will IA45 improve the current IA process?
By streamlining procedures, removing inefficiencies, and fostering collaboration among stakeholders, IA45 will speed up the study activation process without compromising the safety, quality, or integrity of clinical research.
- How can I contact the project team?
For inquiries, please reach out to us via email at IA45@uhn.ca . We would love to hear from you.
- How can I support the project?
Ask questions, seek out information, raise concerns, and have an open mind. We know change can be difficult, and we are focused on ensuring people transition successfully. We are asking that UHN employees pay close attention to any communications from UHN leaders.
- How does UHN plan to support departments through this initiative?




