
Epigenetics: decoding the human genome
“The human genome is highly complex. Every cell in our body has 6 billion letters of DNA, but only 1.5% are found in genes. The vast majority of DNA letters are found in ‘non-coding elements’ of our genome, controlling how genes are used differently across a variety of cell types in our body,” says Dr. Mathieu Lupien, recently elected Fellow of the Royal Society of Canada for his cancer epigenetics research and Chair of Genetics & Epigenetics Program at Princess Margaret Cancer Centre (PM).
To fully understand the complexity of the genome, epigenetics cannot be overlooked. Simply put, epigenetics involves the additional information on top of DNA that guides each cell in our body to use its copy of the human genome in unique ways.
“Cells can accumulate variations in the epigenetic layer, called chromatin variants, that fine-tune cell identity within an individual,” Mathieu explains.
Chromatin is the structure that packages DNA in the cell nucleus. It consists of DNA wrapped around histone proteins, forming nucleosomes. Think of chromatin like a bookshelf where the DNA is stored. Just like how tightly or loosely books are arranged can affect how easily you can access them, the structure of chromatin affects whether genes are ‘open’ or ‘closed’ to the cell’s reading machinery.When DNA is accessible in one cell type but not in another due to differences in chromatin compaction, this phenomenon is referred to as chromatin variants.
Most chromatin variants affect the non-coding regions of the genome, which harbours all the information to fine tune gene expression across each cell type in the human body. For instance, the non-coding regions include promoters, which are DNA sequences upstream of genes, act like light switches to turn genes “on” for expression. Enhancers are another example of non-coding regions found far from genes, that function like light dimmers, coordinating with promoters to adjust gene expression levels. However, how chromatin variants impact these regions of the genome to favour cancer development remains a largely unexplored and mysterious area.
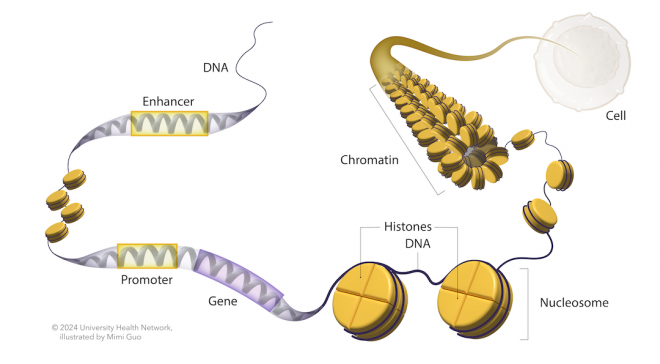
DNA organization inside the cell.
Cancer can arise when chromatin variants accumulate, disrupting normal gene function and promoting uncontrolled cell growth. From Mathieu’s earliest study published in Cell, the team was the first to identify a type of chromatin variant that causes a DNA reading protein, FoxA1, to contribute to both breast cancer and prostate cancer development.
FoxA1 can recognize distinct stretches of DNA because of this type of chromatin variation, present in both cancer types. Those stretches were identified to be enhancers that regulate the gene expression specific to either breast or prostate cancer.
Mathieu moved his lab to PM in 2012 to tap into the local expertise in cancer stem cells. He collaborated with Dr. John Dick and Dr. Peter Dirks to use epigenetics to explore the underlying mechanisms of cancer renewal properties. Since then, he contributed to systematically indexing the genome of normal and cancer cells to build an encyclopedia of non-coding DNA elements across the human genome to unveil cancer-associated epigenetic variation in diverse cancer types, including blood, brain, prostate, breast and colon cancers.
Packing DNA differently: a new driver of cancer and a new therapeutic area
“There has been a research focus on genetic mutations as a cause of cancer,” says Mathieu. “But more and more evidence is also showing how cancer can be a disease of the chromatin.”
Chromatin variant in pediatric ependymoma
Collaborating with Dr. Michael Taylor at SickKids, Mathieu’s team helped identify the first instance of a cancer type, pediatric ependymoma, arising from chromatin variants independently of genetic mutations.
Ependymoma is a type of cancer found in the nervous system and it is more frequently diagnosed in children than in adulthood. With fewer years to be exposed to carcinogens, children accumulate fewer mutations in their tumours compared to adult cancers, making it a puzzling case to understand their origins.
Tapping into the expertise of a team of epigenetics scientists including Mathieu, pediatric ependymomas were found in a study to stratify into two groups based on chromatin variants, revealing clear variations in epigenetics. Further investigation linked these patterns to environmental factors and prompted a clinical trial to test the effectiveness of epigenetic therapy, a new class of treatment options targeting chromatin variants.
Chromatin variant in triple-negative breast cancer
Studying the therapeutic resistance in breast cancer, Mathieu’s team discovered a new class of chromatin variants in chemo-resistant cancer cells, suggesting that this particular class might be treatable with epigenetic therapy.
Together with Dr. Cheryl Arrowsmith and the Structural Genomics Consortium, the team tested a series of chemical compounds in preclinical settings and showed that a specific subset of these compounds can inhibit tumour growth. Mathieu now collaborates with Dr. David Cescon, a Medical Oncologist and Clinician Scientist at PM, to determine the best way to bring epigenetic therapy into the clinic.
Innovating through strategic collaboration
Mathieu was named Allan Slaight Collaborator of the Year in 2022 and has made significant strides in the field of epigenetics through his collaborative efforts.
“Collaboration is the primary source for innovation,” says Mathieu. “We innovate by merging different fields together. And by collaborating with field experts, we can avoid going blindly into a new space.”
Mathieu emphasizes the importance of strategic collaboration in academia. “Before collaborations, one needs to establish their own value proposition clearly—what you’re good at and where you need complementary expertise to accomplish what you want. Then you can engage in fruitful collaborations.”
Looking to the future, Mathieu is optimistic about the integration of epigenetics into clinical practice. “What I look forward to in the future is for the field of epigenetics to be embedded within clinical practice. Today, I’m a researcher but I am also the friend, the relative of a cancer patient. I hope that the discoveries my team is making today can inform of a new era of clinical practices.”
“It’s heading in the right direction, and hopefully, we’ll see epigenetic research transform the clinic soon.”
Meet PMResearch is a story series that features Princess Margaret researchers. It showcases the research of world-class scientists, as well as their passions and interests in career and life—from hobbies and avocations to career trajectories and life philosophies. The researchers that we select are relevant to advocacy/awareness initiatives or have recently received awards or published papers. We are also showcasing the diversity of our staff in keeping with UHN themes and priorities.




