
An Unwavering Passion for Cancer Immunology
Naoto Hirano was born in Japan. In his early years, his family stayed in the United States for his father’s medical career for a few years. This brief time abroad left a lasting impression on him, shaping his future path. Deliberating between law and medicine, Naoto decided to pursue medical school, influenced by his father's successful career and his admiration for him.
During his time as a medical student at the University of Tokyo, Naoto discovered his passion for immunology while working in an immunology lab. He was also thrilled to work in the same lab as Yusuke Yanagi, a co-author of a seminal 1984 Nature paper that reported the first T cell receptor cloning by Tak Mak at the Ontario Cancer Institute (the former name of the research arm at Princess Margaret Cancer Centre).
While choosing a medical specialty, Naoto consulted a professor in immunology about pursuing studies in tumour immunology. Rather than encouragement, he was discouraged from this path. Instead, the professor suggested transplantation immunology, which was a more evolved field at that time.
Naoto revised his plan and chose internal medicine for his residency. While fulfilling his clinical responsibilities, he also conducted research on genes that drive cancer, called oncogenes. “It was a crazy time—I was working 24/7, and travelling between hospitals; however, I gained rich skillsets in molecular biology. After completing my training, I needed to consider what to do next.”
Naoto was given the opportunity to continue research on oncogenes but declined the offer. “I said ‘no’ because I was still keenly interested in tumour immunology.” At the time, the B7 costimulatory molecule—an important molecule in cancer immunotherapy—was just discovered. Naoto realized he needed to seize the opportunity to contribute to this burgeoning field.
His mentor, Dr. Hisamaru Hirai supported his decision to study cancer immunology and immunotherapy, and convinced him to stay in Tokyo. In the lab, he found it relatively simple to treat experimental mouse models of cancer, but at the same time he would see cancer patients pass away in the clinic. He realized that there was something wrong with the model systems he was using and that working with more clinically relevant models would have more impact. “I determined that it was important to perform immunology experiments using human-derived experimental models,” says Naoto.
In 1996, due to regulations in Japan, experiments with human samples were restricted. Seeing this roadblock, Naoto recalled his early years abroad and wondered if he could better advance the field from somewhere else. He applied for positions at five or six institutions all over the world and eventually secured a fellowship award and position at the Dana-Farber Cancer Institute in Boston in the lab of Dr. Lee Nadler. It was there that Naoto was finally able to consider clinical and human samples—and began the work that marked the start of his career as an independent investigator in Boston.
However, three years later, when Naoto wanted to expand his program on T cell-based cancer immunotherapy research, he was turned down. Dissatisfied, but not discouraged, Naoto was determined to make it happen. “I just had to change my path again. I started job hunting to find a place that would support my research interests.”
Naoto heard through the grapevine that the Princess Margaret Cancer Centre was looking for someone who studies cancer immunotherapy in humans. The Director of the centre at that time, Dr. Benjamin Neel, invited Naoto to an interview with him and other scientists Drs. Tak Mak and Pam Ohashi.
“I knew that the field of immunology here was very strong and that it would be a great place for me. I accepted the offer. I started at the Princess Margaret as a Scientist and as the Associate Director of research at the centre’s Tumour Immunology Program; I was also appointed an Associate Professor in Immunology at the University of Toronto. Everyone was working collaboratively on T cell biology research, and I was eager to combine my strengths in molecular biology with immunology to discover something that can be translated to the clinic.”
Making Immunotherapies Accessible to All
A promising and emerging treatment approach that Naoto is studying is known as chimeric antigen receptor (CAR) T cell therapy. T cells are an important component of our immune system that find and destroy defective cells. CAR T cell therapy uses these cells by genetically modifying them so that they recognize and kill cancer cells.
CAR T cell therapy involves modifying a patient’s own T cells in the lab by inserting CAR genes, which can then recognize and target specific proteins or sugars called antigens on a cancer cell’s surface. These CAR T cells are then grown and multiplied in the lab before they are eventually infused back into the patient to fight cancer.
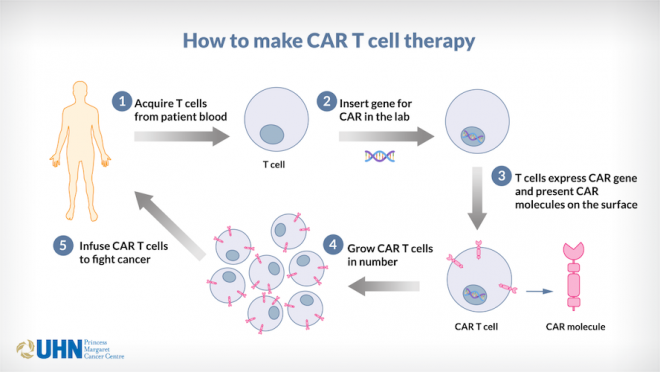
Illustration showing how CAR T cell therapy works.
However, the full potential of CAR T cell therapy has not yet been fully realized in the clinic. These T cells still need to be activated by cytokines, which are proteins that circulate through the body and help to facilitate immune cell signaling. This activation triggers a series of biochemical reactions inside the cell—involving JAK and STAT proteins—to enhance the ability of T cells to grow and kill cancer.
While cytokines can activate CAR T cells, at excessive levels cytokines can induce strong adverse events in some individuals. So how do we activate T cells while bypassing unwanted side effects?
To address this issue, Naoto’s team developed an improved version of CAR T cells—called JAK-STAT CAR T cells. These cells contain a new CAR molecule, which has two additional protein domains linked to it. These portions of the molecule mimic cytokine receptors and recruit JAK and STAT proteins, thereby activating the cells.
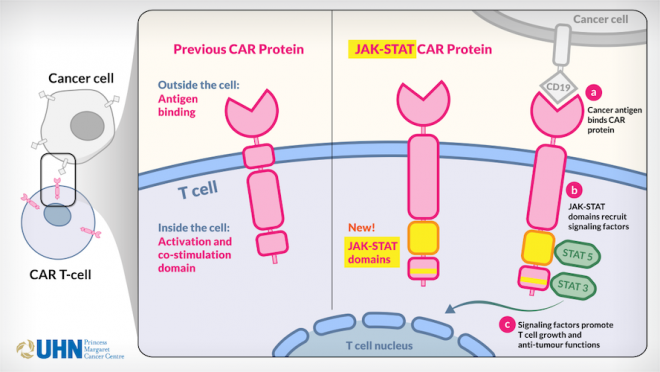
Illustration showing how Naoto's JAK-STAT CAR T cell works.
The performance of JAK-STAT CAR T cells has shown better anti-tumour effects in both in vitro and in vivo assays than regular CAR T cells. Naoto’s team published the results in Nature Medicine in 2018 (news story here) and patented the technique. Since then, the team has paired up with the biotech company Takara Bio to test its effectiveness in preclinical settings and apply for clinical trial approval. In 2023, the team received approval from Health Canada and announced the initiation of a first-in-human clinical trial for JAK STAT CAR T-cell therapy.
“It’s a completely new generation of CAR T cell therapy, which has the potential to enhance the effectiveness of the therapy. Researchers are currently studying its viability and limitations,” says Naoto.
Currently, CAR T cell therapies are being used to target blood cancers such as B cell malignancies, but there are still challenges using CAR T cells to treat solid tumours. Naoto thinks that a related approach, known as T-cell receptor (TCR) gene therapy, may be better suited to treat solid cancers.
Unlike CAR T cell therapy, which involves the use of an engineered CAR molecule to target specific antigens on cancer cells, TCR gene therapy involves the selection of naturally occurring T-cell receptors that recognize and target cancer. When targeting cancer, T cell receptors identify fragments of cancer proteins that are presented on special molecules known as human leukocyte antigen (HLA) molecules on the surface of cancer cells.
A current limitation of TCR therapy is that its effectiveness can vary depending on a patient’s racial background. The key issue is the pairing between TCR and HLA—and the fact that HLAs are highly genetically diverse. If a TCR works for patients in one racial group, it may not work in patients from other racial groups that do not have the same peptide-HLA complex on their cancer cell—a limitation that can lead to treatment failure.
“I don’t like that exclusion. I want this therapy option to be available for everyone,” says Naoto.
Naoto’s team developed a patented technology that enables the interaction between a TCR and cancer antigen to be defined for any HLA allele and peptide, allowing the inclusion of patients with rare alleles. This work was published in the journals eLife (HLA class I, 2020) and Nature Biotechnology (HLA class II, 2021).
TCR gene therapies are in their inception—there are few trials that have been completed that show good clinical efficacy. To advance TCR gene therapy, Naoto along with Dr. Tak Mak at PM and Dr. Mark Davis at Stanford University co-founded the TCR-T cell-based immunotherapy company TCRyption Inc. in 2020, with the help of UHN Commercialization. The company was later acquired by Treadwell Therapeutics in 2021.
When asked about his advice on doing research that has commercialization value, Naoto says, “People think that patent filing and commercialization depends on serendipity, but I don't think so. When I initiate a project, I always decide from early on what the final output should be.”
Embracing Changes
Driven by a thirst for knowledge, and a belief in following one’s dreams, Naoto has consistently sought out environments that fuel his ambition and offer the freedom to explore through research. "If it's not available where I am, I don't hesitate to seek it elsewhere," he affirms. His journey has taken him from the bustling streets of Tokyo to the vibrant cities of Boston and Toronto—different environments that offer unique opportunities for growth and exploration.
“Young people shouldn't feel obligated to making changes to their environment; it's a costly and time-consuming task. Instead, focus on finding a place that aligns with your goals and aspirations. Embrace the world outside, take the risk to move around,” Naoto asserts. “And it's never too late to come back if you feel your roots pulling you back.”
Naoto's perspective is not solely based on his own journey but also on that of his son. "My son left Japan to pursue a second degree in music to master the cello. After realizing his passion for music, he has now returned to the field of consulting. Embracing change is important for happiness."
Meet PMResearch is a monthly column that features Princess Margaret researchers. It showcases the research of world class scientists, as well as their passions and interests in career and life—from hobbies and avocations to career trajectories and life philosophies. The researchers that we select are relevant to advocacy/awareness initiatives, or have recently received awards or published papers. We are also showcasing the diversity of our staff in keeping with UHN themes and priorities.

