
Dr. Daniel De Carvalho was on a plane, about to depart for a conference, when his phone rang—a call from the Gairdner Foundation. Three hours later when he landed, he answered the call to learn the surprising news: The Gairdner Foundation was awarding him the 2025 Peter Gilgan Canada Gairdner Momentum Award.
“I was so excited,” said Daniel of the award, which goes to mid-career investigators for exceptional scientific research contributions. “The most rewarding part of receiving this award is that it renews our energy and focus to keep translating our discoveries into new diagnostics and therapies. It’s incredibly meaningful to see the work starts to make a difference for our patients and society.”
Dr. De Carvalho, a global leader in cancer epigenetics, and his team opened up a new avenue for cancer therapy through their work. He discovered the role of repetitive elements in our genome, in regulating viral mimicry response in cancer cells and provided the rationale for many cancer treatments undergoing clinical trials worldwide. The award also recognizes Dr. De Carvalho’s game-changing development of a novel blood-based test for early cancer detection, classification, and therapy monitoring, poised to revolutionize cancer management.
Viral mimicry: a breakthrough mechanism for anti-cancer therapeutics
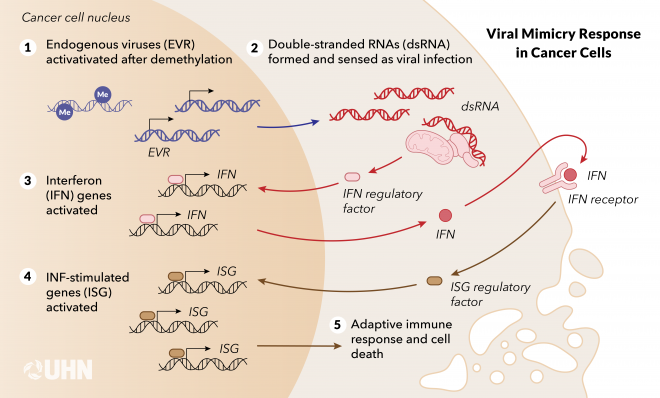
In 2015, Daniel’s team revealed that by activating parts of our own DNA that have an ancient root acquired from a viral infection, cancer cells can be disguised as viruses, triggering an immune response to fight cancers. They named this phenomenon the “viral mimicry”response.
The human immune system is well-prepared to fight viruses, but it struggles to recognize cancer cells. The discovery of viral mimicry made it possible to design better therapies that fight cancer through an antiviral route.
The team found double-stranded RNAs (dsRNA) in colorectal cancer cells, following the treatment with an epigenetic drug. dsRNA is a molecular structure typically present in host cells infected by viruses and an intermediate for viral replication.
“We saw a very strong antiviral response, but there was no viral infection,” says Daniel. These dsRNA fragments triggered a series of reactions that caused the cells to produce and secrete interferons, an antiviral signal to activate the immune system and destroy cancer cells.
But where did they come from if there was no virus in the first place? The study discovered that those dsRNAs were made from repetitive elements in the human genome called endogenous retrovirus—remnants of ancient retroviral infections that have become permanently integrated into our DNA. Unlike active retroviruses such as HIV, endogenous retroviruses no longer cause infection; instead, they are inherited like regular genes and passed down through generations.
The epigenetic drug used to elicit the viral mimicry response removes methyl groups, a chemical mark, on DNA. By demethylating DNA in the cancer cells, the drug unmasked endogenous retrovirus sequences that have long been silenced and domesticated in the genome, inducing a high level of dsRNAs in the cancer cells and an antiviral response. With more studies followed in the field, this discovery fundamentally changed our understanding of the physiological role of repetitive elements in the human genome and their function in anti-cancer therapies.
The team’s landmark paper was published in Cell, it was selected as one of the top 10 notable advances of 2015 by Nature Medicine, and was highlighted in the New England Journal of Medicine’s ‘Clinical Implications of Basic Research’ section.
Since then, the team has been working on pharmacologically manipulating this mechanism, to induce a strong antiviral response, for a stronger, anti-tumour therapy. Their discoveries have led to a patent filing and joint drug discovery program with pharmaceutical companies to develop novel anti-cancer therapies.
The viral mimicry state induced by epigenetic therapy (adapted from Cancer Discovery, 2021).
Surprise is the strongest signal of something new
For Daniel, the discovery of viral mimicry response was a total surprise. At the time, scientists had observed the anti-cancer effects of some epigenetic drugs in clinical trials, but they didn't know why they worked.
"Our hypothesis was that the DNA demethylating drug activates tumour suppressor genes," Daniel recalls. "We absolutely were not expecting an antiviral response."
All the immune cells have the same genetic information, but they have very different functions. Different genes need to be activated or repressed in different cells to have a diversity of functions. This led me to the field of epigenetics, and later cancer epigenetics.”
Daniel earned his PhD study in Immunology at the University of São Paulo and completed a postdoctoral fellowship at the University of Southern California before moving to Toronto. He established his team at UHN’s Princess Margaret Cancer Centre and began a faculty position at the University of Toronto in 2012.
He feels a great sense of fulfilment and purpose in his research journey. “It's about enjoying the journey and process of doing science, not the destination. A lot of times, you can hit into dead ends, but sometimes you get to an interesting place. Surprise is always the strongest signal that you have found something new.”
Racing against time: early cancer detection, classification, and interception
It’s hard to treat cancer when a patient has an advanced disease. Another focus of Daniel’s group is to leverage epigenetic changes for early cancer detection, patient classification, cancer prevention, and interception.
“Compared to the normal human genome, cancer patients show massive changes in DNA methylation profiles. This creates opportunities to detect cancer early,” says Daniel.
His lab developed cfMeDIP-seq (cell-free Methylated DNA Immunoprecipitation and high-throughput sequencing), a highly sensitive method that isolates and sequences methylated DNA fragments in the blood. This method is revolutionizing the use of liquid biopsy, enabling a single blood sample to detect cancers in a cost-effective way. Unlike traditional methods, which require prior knowledge of a patient’s tumour-specific genetic mutations, cfMeDIP-seq identifies universal epigenetic changes shared across many cancers.
This next-gen approach was published in Nature in 2018, uncovering many methylated regions in plasma cfDNA from patients with different cancer types, even in early-stage disease. The method has shown robust cancer detection in seven different cancer types, including clinical evidence of detecting and classifying kidney cancers and intracranial tumours in two back-to-back publications in Nature Medicine.
Daniel, with Dr. Scott Bratman of PM and Dr. Anne-Renee Hartman, Maneesh Jain, and David Scheer, co-founded Adela, a biotech company, with the support of Commercialization at UHN and institutional investors. Their blood test was clinically validated and can detect head and neck cancer relapse up to 14.9 months earlier than the standard of care procedures, published in The Annals of Oncology.
Beyond detection, Daniel’s research has opened doors to cancer interception—stopping cancer before it fully forms. His viral mimicry study revealed that in premalignant lesions, cells that have yet to fully become cancerous can activate transposable elements, triggering a chronic immune response that eventually leads to immune exhaustion and tumour development. This work was published in Cancer Discovery and his team has explored using drugs to block this process, delaying or even preventing cancer formation in preclinical models.
"I'm really excited to deepen our understanding of how cancer initiates, which will lead to better therapies and interception strategies. In the field of liquid biopsy, I look forward to advancing the technology to make it more sensitive and specific, and to testing it in larger populations."
Meet PMResearch is a story series that features Princess Margaret researchers. It showcases the research of world-class scientists, as well as their passions and interests in career and life—from hobbies and avocations to career trajectories and life philosophies. The researchers that we select are relevant to advocacy/awareness initiatives or have recently received awards or published papers. We are also showcasing the diversity of our staff in keeping with UHN themes and priorities.




