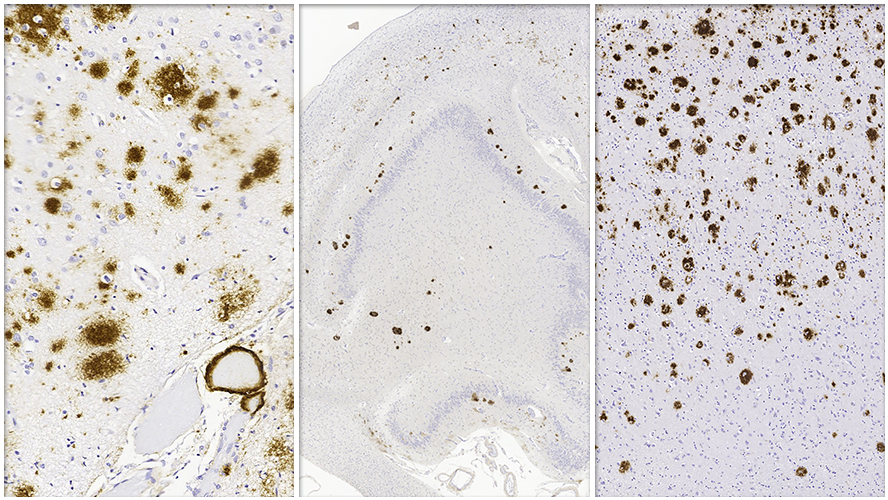
Alzheimer disease (AD) is marked by pathological changes in the brain, collectively known as Alzheimer disease neuropathological change (ADNC). Typical ADNC is defined by a build-up of amyloid-beta proteins (called amyloid plaques) and tau proteins (called neurofibrillary tangles). Despite advances in research, defining subtypes of ADNC and predicting related patient outcomes remain significant challenges for clinicians and scientists.
A recent study, co-led by a team at the Krembil Brain Institute at UHN offers new insights into how we could overcome this challenge. The study identified distinct genetic and clinical features across ADNC subtypes, focusing on a newly proposed subtype called amyloid-predominant ADNC (AP-ADNC).
The team found that AP-ADNC has unique genetic traits that may be protective against tau build up and influence disease progression differently compared to other ADNC subtypes. Previously, this subtype was thought to be a separate condition unrelated to Alzheimer disease. By recognizing AP-ADNC as part of the ADNC continuum, the researchers highlighted an opportunity to redefine how these subtypes are classified and understood.
The findings not only expand the understanding of ADNC diversity but also lay the groundwork for more personalized approaches to care. Subtypes like AP-ADNC may require different diagnostic and therapeutic strategies, reflecting their distinct pathways of progression.
These results emphasize the need to further explore the links between ADNC subtypes and cognitive decline. The ultimate goal is to predict patient outcomes more accurately and develop tailored treatments based on specific subtype characteristics.
As researchers delve deeper into the complexities of ADNC, studies like this bring the medical community closer to personalized care for individuals with Alzheimer disease. Recognizing the diverse pathways underlying the condition is essential for improving patient outcomes and advancing therapeutic approaches.
The first author on this study is Dr. Gabor Kovacs, a Senior Scientist at Krembil Brain Institute and Professor in the Department of Laboratory Medicine and Pathobiology at the University of Toronto.
Dr. Shelley Forrest, Senior Scientific Associate at Krembil Brain Institute, also contributed to the study.
The senior author of this study is Dr. Peter Nelson, Professor in the Department of Pathology at the University of Kentucky.
This work was supported by the National Institutes of Health, National Institute on Aging (NIH-NIA), and UHN Foundation.
Kovacs GG#, Katsumata Y#, Wu X, Aung KZ, Fardo DW, Forrest SL; Alzheimer's Disease Genetics Consortium; Nelson PT. Amyloid-β predominant Alzheimer's disease neuropathologic change. Brain. 2024 Oct 17:awae325. doi: 10.1093/brain/awae325. Epub ahead of print.
#Contributed equally




