
A research team at the Princess Margaret Cancer Centre (PM) has uncovered the three-dimensional structure of a key region of a drug target in cancer, diabetes and fatty liver diseases.
The target—known as fatty acid synthase (FASN)—is an enzyme that is involved in the production of a fatty acid called palmitate, which plays a role in fat metabolism and communication within the cell.
“A deeper understanding of the structure and mechanism of FASN inhibition could unlock next generation therapeutics for a variety of solid tumours," explains Naimul Hasan, a graduate student in Dr. Mazhab-Jafari’s lab and first author of the study.
To date, no study has defined the structure of the specific region of FASN where therapeutic inhibitors bind, including the clinically available Denifanstat inhibitor. This gap in knowledge is key to facilitating drug testing and development.
To address this, the team took a new approach. Unlike previous studies, which only engineered and analyzed shorter sections of the protein, the team engineered a full-length version of human FASN. “Working with the full-length protein is important because, in this form, it can interact normally with cellular components and be chemically modified as it would in nature,” says Dr. Mazhab-Jafari, Scientist at PM.
After producing the protein, the team cut it up into subdomains using strategically positioned cleavage sites that were engineered in. They then isolated the region of FASN that binds to Denifanstat using protein purification methods and determined the structure of the region using an advanced imaging technique called cryogenic electron microscopy (cryoEM). This approach enabled them to determine the structure of the protein fragment at the near atomic resolution of 2.4 angstroms (i.e., 2.4 ten-billionths of a metre).
“Our research has unraveled the structural intricacies of the FASN enzyme and provided crucial insights into its behavior. Equipped with this knowledge, we can now design drugs with greater precision to specifically target FASN and combat a variety of diseases," adds Dr. Mazhab-Jafari.
This work was supported by the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Ontario Government, the Canada Foundation for Innovation and The Princess Margaret Cancer Foundation.
Hasan SMN, Lou JW, Keszei AFA, Dai DL, Mazhab-Jafari MT. Atomic model for core modifying region of human fatty acid synthase in complex with Denifanstat. Nat Commun. 2023 Jun 12;14(1):3460. doi: 10.1038/s41467-023-39266-y.
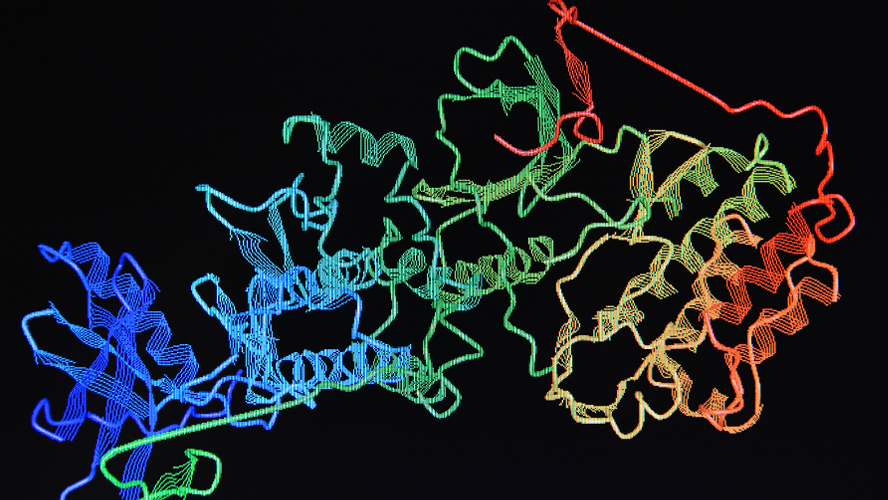
Using advanced imaging techniques, researchers are able to define the intricate three-dimensional structure of proteins. Illustrated above is the molecular structure of a human enzyme.




