Have you ever touched a hot surface? Almost instantly, your brain not only receives messages about heat and pain, but also sends a message to pull your hand away from the surface.
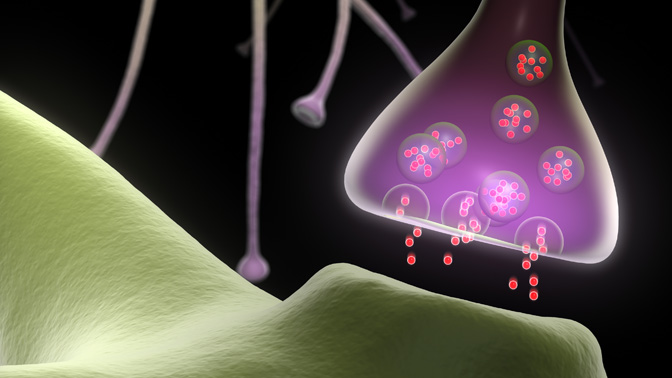
These messages are transmitted across a complex network of specialized cells, called neurons, in a matter of milliseconds. This rapid method of communication is accomplished using small chemical messengers called neurotransmitters: the neuron sending the message releases neurotransmitters that are received by the next neuron, which continues to pass the message along in the same way.
Inside the sending neuron, neurotransmitters (ie, the message) are stored within “synaptic vesicles” (ie, the envelope). Synaptic vesicles fuse to the inner wall of the neuron nerve fiber terminal and rapidly release the neurotransmitters enclosed within, passing them on to the target neuron; however, it remains unclear how this function is accomplished.
It is for this reason that Krembil Senior Scientist Dr. Elise Stanley and her research team recently initiated a study to determine the exact interactions between synaptic vesicles and the sending neuron's inner wall. It is known that proteins embedded in the neuron’s wall, called voltage-gated calcium channels (CaVs), enable synaptic vesicles to fuse. Thus, the team used sophisticated techniques to label synaptic vesicles and CaVs; they then visualized these labelled structures with state-of-the-art electron microscopes. The team found that CaV proteins anchor synaptic vesicles in place, right next to the neuron’s inner wall. This CaV-synaptic vesicle binding could be the critical first step in triggering neurotransmitter release: the CaV may grab synaptic vesicles floating within the cell—bringing them close to the cell membrane such that they are ready to release their neurotransmitter contents.
“The results of this study contribute to our fundamental understanding of how cells in the nervous system communicate with one another,” says Dr. Stanley. “By building a more complete picture, we can identify molecules or processes that could be exploited for therapeutic purposes in cases where communication between neurons goes awry, as it does in degenerative or functional brain disorders such as Alzheimer disease or epilepsy.”
This work was supported by the Canadian Institutes of Health Research and the Toronto General & Western Hospital Foundation. E Stanley is a Tier 1 Canada Research Chair in Molecular Brain Science.
Chen RHC, Li Q, Snidal CA, Gardezi SR, Stanley EF. The calcium channel C-terminal and synaptic vesicle tethering: analysis by immuno-nanogold localization. Front Cell Neurosci. 2017 Mar 30. doi: 10.3389/fncel.2017.00085.




