A recent study from the Krembil Brain Institute (Krembil) at UHN has revealed the essential role of the immune system in response to treatments for spinal cord injury (SCI). Comparing different laboratory models of SCI, a research team led by Krembil Senior Scientist Dr. Michael Fehlings discovered that cell therapy outcomes vary significantly between immunocompromised or immunodeficient models—which lack a fully functioning immune system—and immunocompetent models.
Cell therapies, such as human induced-pluripotent stem cell-derived neuron progenitor cell (hiPSC-NPC) transplants, are promising treatments for patients with SCI, many of whom have no other treatment options. However, to ensure cell survival and minimize rejection, preclinical models that scientists use to study the safety and feasibility of cell therapies, often exclude or downplay the immune system’s role. This trade-off affects how well these models replicate the real-world response to treatment.
Findings from this study suggest that this approach limits the relevance of these models to actual patients. Researchers observed that some immunodeficient lab models favour the development of neurons and oligodendrocytes from hiPSC-NPCs at the expense of the development of astrocytes, while astrocytes are the most common cell type that develop in immunocompetent models.
“Immunodeficient models seem to lack the intricate interplay between the transplant recipient’s immune system and the transplanted cells, resulting in an altered ratio of cells compared to what is expected in patients,” explains Dr. Fehlings. Further investigation also found that immunodeficient lab models show lower levels of cell death and stress along with higher levels of nervous system development and cell growth signalling.
Although it is not possible to replicate every aspect of SCI in preclinical models, this work highlights the need for more comprehensive models that better reflect the immune system’s role with transplant survival. Successfully addressing this challenge will bring cell therapies for SCI closer to clinical application than ever before.
The lead author of this study is Dr. Zijian Lou, a graduate research student at the Krembil Brain Institute in the Fehlings Lab.
The senior author of this study is Dr. Michael G. Fehlings, a Senior Scientist at the Krembil Brain Institute at UHN, the Head of the Spinal Program at UHN’s Toronto Western Hospital, and a Professor in the Department of Surgery at the University of Toronto.
This work was supported by Wings for Life, the Krembil Foundation, the Canadian Institutes of Health Research, and UHN Foundation.
Lou Z, Post A, Nagoshi N, Hong J, Hejrati N, Chio JCT, Khazaei M, Fehlings MG. Assessment of immune modulation strategies to enhance survival and integration of human neural progenitor cells in rodent models of spinal cord injury. Stem Cells Transl Med. 2025 Feb 11;14(2):szae090. doi: 10.1093/stcltm/szae090.
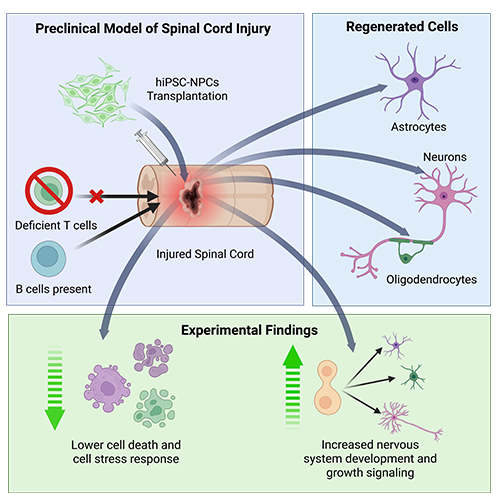
Graphical abstract showing the paper’s main findings. (Image credit: Fehlings Lab, created with BioRender.com)




