 As UHN's monthly research newsletter, NRx reports on the full spectrum of research from UHN's five research institutes: the Princess Margaret (PM) Cancer Centre, the Toronto General Research Institute (TGRI), the Krembil Research Institute (Krembil), the Toronto Rehabilitation Institute (TRI) and the Techna Institute (Techna).
As UHN's monthly research newsletter, NRx reports on the full spectrum of research from UHN's five research institutes: the Princess Margaret (PM) Cancer Centre, the Toronto General Research Institute (TGRI), the Krembil Research Institute (Krembil), the Toronto Rehabilitation Institute (TRI) and the Techna Institute (Techna).
In this issue you can read about:
- A rapid kidney disease test that could eliminate two-year wait
- Why communicating advancements could improve physiotherapy
- A new way that cancers evade the immune system
- Detecting heart damage due to blood transfusions before it's too late
- A three-dimensional view of what happens to arthritic bones
- Studying elusive immune cells that recognize 'self' molecules
- The Michener Institute of Education at UHN
- Federal funding for Toronto cell therapy facility
Christopher J. Paige, PhD, FCAHS
Executive Vice President, Science and Research
University Health Network
A new test eliminates the two-year waiting period needed to predict the risk of kidney failure
In IgA nephropathy, the kidney's filters are blocked by an accumulation of abnormal IgA antibodies. This image shows normal IgA molecules (in light blue) clearing a virus.
IgA nephropathy is the most common cause of inflammation in the filters of the kidney. Some people with the disease will develop kidney failure, a life threatening condition.
To determine those at risk of developing kidney failure, clinicians review blood pressure and urine protein levels in those with IgA nephropathy over a period of at least two years. Because of the waiting time required for this prediction, effective therapies are often delayed.
To address this issue, TGRI Senior Scientist Dr. Daniel Cattran led a team of international experts to develop a test to predict the risk of kidney failure sooner. The team examined kidney tissue biopsy samples from those diagnosed with IgA nephropathy and they evaluated the cellular features in the samples according to a set of criteria known as the MEST score. They found that the combination of the MEST score and other clinical features collected at the same time could predict which patients would develop kidney failure, and that the approach was as accurate as using the data gathered over two years of follow-up.
"With earlier prediction of which patients will develop kidney failure, our new approach enables this patient group to be treated two years sooner," explains Dr. Cattran. "This in turn will help to prevent or slow the progression of their kidney disease."
This work was supported by the Carraresi Foundation, the Michael Smith Foundation for Health Research, the European Renal Association – European Dialysis and Transplant Association, the International IgA Nephropathy Network, the Toronto GN Registry and the Toronto General & Western Hospital Foundation.
The MEST score provides earlier risk prediction in lgA nephropathy. Barbour SJ, Espino-Hernandez G, Reich HN, Coppo R, Roberts IS, Feehally J, Herzenberg AM, Cattran DC; Oxford Derivation, North American Validation and VALIGA Consortia; Oxford Derivation North American Validation and VALIGA Consortia. Kidney International. 2016 Jan. [Pubmed abstract]
Improving physical therapists' access to resources may enhance stroke rehabilitation programs

Stroke is the leading cause of adult disability. In 2010, the worldwide prevalence of stroke was 33 million; 16.9 million of these people experienced a stroke for the first time.
A stroke can cause weakness or paralysis on one side of the body; it may affect the whole side or just the arm or leg. Functional electrical stimulation (FES) is a therapy that delivers repeated electrical shocks to stroke survivors' affected limbs and has been shown to accelerate recovery. Despite its effectiveness, and that it is widely recommended in best practice guidelines, little is known about whether FES is actually being used in stroke rehabilitation programs.
Recently, TRI Scientist Dr. Kristin Musselman and her team distributed an online survey to physical therapists across Canada to determine how often physical therapists incorporate FES as part of their stroke rehabilitation programs. It included questions that measure the therapists' demographic characteristics, FES use, knowledge of FES literature and perceived obstacles to FES use.
Results from the survey revealed that the majority of physical therapists reported never or rarely using FES. However, over half of the 298 therapists who responded said that they would like to use it more. A lack of access to resources, including time, equipment and training, was the most frequently cited barrier to FES use.
"Our findings suggest that physical therapists are more likely to use FES if they have received hands-on training and guidance on how and when to apply it," says Dr. Musselman. "Improving access to resources, notably continuing education, may facilitate the implementation of FES into clinical practice, and ultimately accelerate the recovery of stroke survivors in Canada."
This work was supported by the School of Physical Therapy, University of Saskatchewan.
Physical therapists' use of functional electrical stimulation for clients with stroke: frequency, barriers, and facilitators. Auchstaetter N, Luc J, Lukye S, Lynd K, Schemenauer S, Whittaker M, Musselman KE. Physical Therapy. 2015 Dec 23. [Pubmed abstract]
UHN researcher discovers new mechanism used by cancer to evade the immune system
Dendritic cells (purple cell illustrated above) act as sentinels for the immune system: they travel throughout the body searching for threats such as disease-causing microbes.
Tumours can suppress the immune system. They can secrete molecules that dampen the function of immune cells in the tumour's immediate environment. This strategy helps cancer evade the immune system and grow unabatedly. TGRI Senior Scientist Dr. Mark Cattral has recently identified a new pathway through which these molecules can inhibit the function of two key mediators of the immune response: dendritic cells and T cells.
Dr. Cattral's research team demonstrated that tumours secrete molecules that activate the protein Toll-like receptor 2 (TLR2) in dendritic cells. The activation of the TLR2 protein and its pathway dampens the anti-tumour activity of dendritic cells and T cells. In an experimental model, the researchers showed that blocking tumour-mediated TLR2 activation not only enhanced the anti-tumour activity of both cells, but also promoted the proliferation of T cells and boosted the effectiveness of other cancer therapies, including a cancer vaccine.
Dr. Cattral's findings could lead to the development of new therapeutic strategies that could be administered in combination with existing cancer therapies to improve their efficacy and clinical outcomes.
This work was supported by the Canadian Institutes of Health Research, the Canadian Cancer Society, Astellas Pharma Inc., the Toronto General Hospital Transplant Program and the Toronto General & Western Hospital Foundation.
Toll-like receptor 2 activation promotes tumor dendritic cell dysfunction by regulating IL-6 and IL-10 receptor signaling. Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS. Cell Reports. 2015 Dec 29. [Pubmed abstract]
MR Imaging can be used to identify early stages of heart damage from iron overload
The heart muscle can be damaged from excess iron that builds up after repeated blood transfusions needed to treat conditions like thalassemia major.
Thalassemia major is a hereditary condition where people lack the ability to produce normal hemoglobin—the oxygen-carrying molecule in blood—causing chronic fatigue. It requires regular blood transfusions to treat. A common side effect of these transfusions is an overload of iron in the body, which can damage the heart and other organs. Magnetic resonance imaging (MRI) is being used clinically to monitor for potential iron overload with a specific focus on the liver and the heart; finding such an overload may trigger initiation of or changes in chelation therapy.
A recent study by Techna Affiliated Faculty Dr. Bernd Wintersperger and colleagues used magnetic resonance imaging (MRI) to further examine the hearts of patients with thalassemia major in detail. MRI enabled the researchers to take readings for what is known as the extracellular volume (ECV) fraction, a measure that reflects the amount of space outside the muscle cells in the heart. In various cardiac pathologies ECV has been shown to reflect the degree of subtle diffuse scaring of the heart muscle; such changes are associated with further damage of the muscle resulting in impaired cardiac function and potentially life-threatening heart rhythm irregularities. In this study, patients with a history of iron overload had a higher ECV measurement—indicative of early damage—while patients without prior iron overload or healthy individuals did not.
Heart failure is one of the major causes of death in patients with iron overload, and once damage to the heart begins it cannot be reversed. Early detection of damage with MRI will allow medical care teams to better manage iron levels in patients with thalassemia major and other conditions that require regular blood transfusions to help prevent damage to the heart. These findings suggest that MRI could serve as an effective way to monitor for the earliest signs of damage.
"We clinically apply MRI techniques that let us measure iron overloads in the heart, but those overloads may be reversible by therapeutic interventions. However, this technique provides us further non-invasive insight into resulting damage of the heart muscle which may currently may not be reversible. This may potentially result in earlier triggers for intervention," comments Dr. Wintersperger.
This work was supported by the Radiological Society of North America, the Toronto General & Western Hospital Foundation and by non-financial research support from Siemens.
Quantification of myocardial extracellular volume fraction with cardiac MR imaging in thalassemia major. Hanneman K, Nguyen ET, Thavendiranathan P, Ward R, Greiser A, Jolly MP, Butany J, Yang IY, Sussman MS, Wintersperger BJ. Radiology. 2015 Dec 10. [Pubmed abstract].
Advanced imaging used to precisely define bone changes in those with ankylosing spondylitis
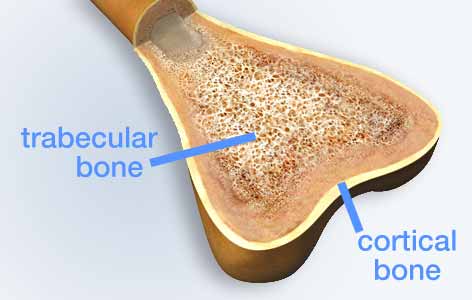
Bone is made of two types of tissue: compact, cortical bone, which provides structural support to the body, and spongy, trabecular bone.
Ankylosing spondylitis is a debilitating form of arthritis that mainly affects the spine. As the disease progresses, complex changes in bone architecture occur. At early stages, the density of the minerals in the spine (ie, bone mineral density) is reduced, while at later stages the outer parts of the vertebrae thicken and fuse together.
To study disease progression, much of the existing research has relied on the use of X-ray images (radiographs) to measure changes in bone. However, because radiographs are two-dimensional, they are unable to show the differences between the harder outer bone and the spongy inner bone.
A recent study, led by TGRI Senior Scientist Dr. Angela Cheung along with Krembil Senior Scientist Dr. Rob Inman and TGRI Scientist Dr. Janet Raboud, used newer, high-resolution imaging to analyse the changes to bone architecture in three dimensions. Their findings revealed that in those with ankylosing spondylitis, the harder outer bone in certain skeletal regions had reduced thickness and reduced bone mineral density when measured in three dimensions. These regions were also found to be more porous. Surprisingly, no changes were found in the spongy inner bone.
"While previous findings have suggested that ankylosing spondylitis is associated with weakening of the inner, spongy trabecular bone, our results suggest that an important feature of the disease is that the compact, outer cortical bone becomes compromised. Because cortical bone is weight bearing, these results may explain why those with the disease are at increased risk of vertebral fractures," explains Dr. Cheung.
These findings provide much needed insight into changes that occur in the bones of those with ankylosing spondylitis that will inform the development of new ways of diagnosing and treating the disease.
This work was supported by Amgen Canada and the Canadian Institutes of Health Research. AM Cheung holds a Tier 1 Canada Research Chair in Musculoskeletal and Postmenopausal Health.
Alterations of bone mineral density, bone microarchitecture and strength in patients with ankylosing spondylitis: a cross-sectional study using high-resolution peripheral quantitative computerized tomography and finite element analysis. Nigil Haroon N, Szabo E, Raboud JM, Mcdonald-Blumer H, Fung L, Josse RG, Inman RD, Cheung AM. Arthritis Research & Therapy. 2015 Dec 24. [Pubmed abstract]
Researchers develop a new approach to study a rare, self-reactive immune cell population
T cells develop in the thymus (depicted above), a butterfly-shaped gland located close to the heart.
T cells are immune cells that use surface proteins called T cell receptors to recognize and attack foreign substances.
Certain T cells, known as invariant natural killer T cells (iNKT cells), have receptors that are self-reactive: they recognize the body's own tissues. Although self-reactive cells can attack the body and cause autoimmune disease, it is not currently known why iNKT cells are self-reactive as a part of their normal behaviour. Part of the reason that these cells have been so elusive is that they are found very rarely in the body.
To address this issue, PM Cancer Centre Senior Scientist Dr. Naoto Hirano and his team of researchers devised an innovative two-step process to generate a large number of iNKT cells. First, the team isolated a region of the T cell receptor gene that is common to most iNKT cells, and inserted the region into regular T cells—partially turning them into iNKT cells. To complete the process, they used molecular 'baits' containing proteins similar to self-proteins to attract and expand the population of self-reactive iNKT cells.
Using this pool of generated iNKT cells, the researchers analyzed the protein sequence of iNKT cell receptors and identified three characteristics that are common to those that are highly self-reactive.
"We have developed a novel method that will enable more in-depth research on this elusive immune cell," says Dr. Hirano. "Moreover, we have identified features of the T cell receptor that provide new insights to the role of self-reactive iNKT cells in maintaining health."
This work was supported by the National Institutes of Health, the Ontario Institute for Cancer Research, The Princess Margaret Cancer Foundation, a Knudson Postdoctoral Fellowship and the Canadian Institutes of Health Research.
CDR3-β sequence motifs regulate autoreactivity of human invariant NKT cell receptors. Chamoto K, Guo T, Imataki O, Tanaka M, Nakatsugawa M, Ochi T, Yamashita Y, Saito AM, Saito TI, Butler MO, and Hirano N. Journal of Autoimmunity. 2015 Dec 31. [Pubmed abstract]
Michener has joined UHN in the first merger between a hospital and health education institute

The Michener Institute for Applied Health Sciences has officially joined UHN in Canada's first-ever merger between a hospital and a post-secondary health education institution. Michener is Canada's only post-secondary institution devoted exclusively to specialized applied health education.
Embedding the Michener within UHN's corporate structure will make it easier to include students in real-world operating rooms, labs and imaging suites for advanced learning, while also making it easier for UHN's health professionals to contribute to curriculum development. The merger will also create more opportunities for real-time continuing education for UHN staff.
Although joining UHN creates opportunities for Michener and UHN's constituent hospitals, it will not mean an exclusive relationship for Michener's students—to gain experience in different care environments, students will continue to have clinical placements at various hospitals and institutions across Ontario.
"With this integration we are creating the premier training site in Canada for applied health sciences professionals," said Dr. Peter Pisters, UHN President and CEO. As part of the merger, Michener will now be known as the Michener Institute of Education at UHN.
$20 M has been by pledged the Prime Minister to establish a Toronto cell therapy facility

In January, $20 million in federal funding was pledged to establish and operate a cell therapy development facility in the MaRS Discovery District in downtown Toronto.
The Centre for Advanced Therapeutic Cell Technologies, to be established and operated by the Centre for Commercialization of Regenerative Medicine (CCRM), is billed as the first cell therapy development facility in the world to use a collaborative approach between research institutions and industry to solve cell therapy manufacturing challenges.
"Regenerative medicine is the future," the Prime Minister told an audience of researchers, healthcare executives and businesspeople at MaRS. "And, not only is it the future, it's a branch of medicine that Canada, and the Province of Ontario, are actually quite good at."
UHN is a founding institutional member of CCRM along with UT, the Hospital for Sick Children Research Institute, McMaster University, Mount Sinai Hospital's Lunenfeld-Tanenbaum Research Institute and the Ottawa Hospital Research Institute.
 Congratulations to Krembil Affiliate Scientist Dr.
Congratulations to Krembil Affiliate Scientist Dr.  TGRI Affiliate Scientist Dr.
TGRI Affiliate Scientist Dr.  PM Cancer Centre's Dr.
PM Cancer Centre's Dr.  Krembil Affiliate Scientist Dr.
Krembil Affiliate Scientist Dr.  Dr.
Dr.