 NRx (formerly known as Net Results Express) is UHN's monthly research e-newsletter. Through NRx you can read about ongoing research at our five research institutes, the Ontario Cancer Institute (OCI), the Toronto General Research Institute (TGRI), the Toronto Western Research Institute (TWRI), the Toronto Rehabilitation Institute (TRI) and the Techna Institute (Techna).
NRx (formerly known as Net Results Express) is UHN's monthly research e-newsletter. Through NRx you can read about ongoing research at our five research institutes, the Ontario Cancer Institute (OCI), the Toronto General Research Institute (TGRI), the Toronto Western Research Institute (TWRI), the Toronto Rehabilitation Institute (TRI) and the Techna Institute (Techna).
In this issue you can read about research in:
- Cancer cell response to drug treatments
- The role of the protein GATA-3 in stem cell self-renewal
- The development of cells in the pancreas
- An intelligent wheelchair system for improved safety
- Modeling blood flow in tumours to improve medical imaging
- Autoimmunity in ankylosing spondylitis
We hope that you will find NRx informative and helpful. If you have feedback or questions, please contact www@uhnresearch.ca.
Christopher J. Paige, PhD, FCAHS
Vice President, Research
University Health Network

Space-filling model of the PTEN protein (blue).
A study published in the journal Science by OCI Senior Scientist Dr. Vuk Stambolic reveals a new function for a protein known as PTEN that may have important implications for how cancer patients with PTEN deficiencies are treated.
PTEN regulates the repair of damaged DNA in the cell by increasing the activity of a protein known as PI3K. Dr. Stambolic and his collaborators made the discovery that cells lacking PTEN are more susceptible to cell death if they are treated with a combination of a DNA-damaging agent and an inhibitor of PI3K. Interestingly, when cells lacking PTEN are treated with a DNA-damaging agent alone, they become more prone to developing alterations in their genome. Alterations in the genome can cause cancer cells to grow faster or become more resistant to cancer therapies.
These studies suggest that patients with cancers that lack PTEN would be less responsive if treated with a DNA-damaging agent alone. Such patients would greatly benefit from more personalized treatment plans that take into account their PTEN deficiency.
This work was supported by the Canadian Cancer Society, the National Institutes of Health, the Ontario Ministry of Health and Long-Term Care and The Princess Margaret Cancer Foundation. B. Raught is a Tier 2 Canada Research Chair in Proteomics and Molecular Medicine, B. Neel is a Tier 1 Canada Research Chair in Signal Transduction and Human Disease and T. Mak is a Tier 1 Canada Research Chair in Inflammation Responses and Traumatic Injury.
Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Bassi C, Ho J, Srikumar T, Dowling RJ, Gorrini C, Miller SJ, Mak TW, Neel BG, Raught B, Stambolic V. Science. 2013 July 26. [Pubmed abstract]
Activated GATA3 functions by entering the nucleus (pink) of the cell, where it controls cellular function and development.
Although the protein GATA-3 is known to control cellular development, its role in the generation of blood cells from hematopoetic stem cells (HSCs) has remained uncertain. A recent study published in Nature Immunology by OCI Senior Scientist Dr. Norman Iscove provides new evidence that GATA-3 plays a role in regulating the function of certain HSC populations.
There are two distinct classes of HSCs, which are based on their biology and characteristics. Long-term multi-potent hematopoietic stem cells (LT-HSCs) can maintain certain types of HSCs and blood cells due to their continued ability to self-renew—to give rise to cells identical to themselves. Intermediate-term HSCs (IT-HSCs) can only sustain specific cell HSC populations for about 12 weeks before cell numbers begin to diminish.
Dr. Iscove and his team were able to demonstrate that under certain conditions GATA-3 is active in LT-HSCs and not IT-HSCs. Moreover, interfering with GATA-3 activity was found to increase the ability of LT-HSCs to self-renew and produce more HSCs. These findings may be useful in developing techniques to increase the numbers of HSCs for use in bone marrow transplantation.
This work was supported by the McEwen Centre for Regenerative Medicine, the Terry Fox Foundation, the Canadian Cancer Society Research Institute, the Canadian Institutes of Health Research, the Princess Margaret Cancer Foundation, The Campbell Family Institute for Cancer Research, the Stem Cell Network and the Ontario Ministry of Health and Long-Term Care.
Gata-3 regulates the self-renewal of long-term hematopoietic stem cells. Frelin C, Herrington R, Janmohamed S, Barbara M, Tran G, Paige CJ, Benveniste P, Zúñiga-Pflücker JC, Souabni A, Busslinger M, Iscove NN. Nature Immunology. 2013 August 25. [Pubmed abstract]
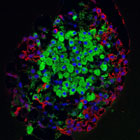
Glucagon-producing α-cells (red) and insulin-producing β-cells (green) are located in regions of the pancreas called the islets of Langerhans.
Pancreatic α- and β-cells together control blood sugar levels through the hormones glucagon and insulin, which have opposing effects. α-cells secrete glucagon to elevate blood sugar levels, while β-cells secrete insulin to lower blood sugar levels. TGRI Scientist Dr. Minna Woo, in collaboration with TGRI Senior Scientist Dr. Eldad Zacksenhaus and TGRI Scientist Dr. Maria Cristina Nostro, examined the exact mechanism through which the developing pancreas balances the proportion of α- and β-cells.
Using an experimental model with pancreatic islet precursor cells lacking the retinoblastoma tumour suppressor protein—an important protein that controls cellular growth—the researchers observed that precursors, normally not present after birth, persisted into adulthood. Islet precursors are cells that develop into the hormone producing pancreatic α- and β-cells. Interestingly, more β-cells were produced from precursors; and α-cells either stopped growing, died or were converted to β-cells. β-cells were also shown to have increased insulin-secreting activity. Ultimately, this change in proportion of α- and β-cells protected the experimental model from disruptions in blood sugar levels and developing diabetes. “This study provides fascinating new insight into the development of the pancreas and offers an innovative strategy for diabetes therapy,” says Dr. Woo.
This work was supported by the the Canadian Institutes of Health Research and the Canadian Diabetes Association. M. Woo is a Tier 2 Canada Research Chair in Signal Transduction in Diabetes Pathogenesis.
Retinoblastoma tumor suppressor protein in pancreatic progenitors controls α- and β-cell fate. Cai EP, Wu X, Schroer SA, Elia AJ, Nostro MC, Zacksenhaus E, Woo M. Proceedings of the National Academy of Science. 2013 August 14. [Pubmed abstract]

Almost half of all older adults in Canadian health care institutions use a wheelchair—the number of such patients is expected to increase as Canada’s population ages.
The safe use of powered wheelchairs can be compromised in older adults with cognitive impairments, who suffer from limitations in attention, reflexes and memory. Without other means of transport, these older adults are left with reduced mobility and independence. To help address this problem, TRI Senior Scientist Dr. Alex Mihailidis has been evaluating a new intelligent wheelchair system (IWS) that was recently developed at the University of Toronto’s Intelligent Assistive Technology & Systems Lab, in collaboration with researchers at the University of British Columbia through the CanWheel Emerging Team funded by the Canadian Institutes of Health Research.
The IWS system provides powered wheelchairs with an anti-collision feature, which helps prevent them from running into obstacles, and a navigation assistance feature, which plays audio prompts to help users maneuver around objects. Dr. Mihailidis and his team found the IWS system to be effective at preventing collisions and navigating around obstacles in simulated environments. The system was also effective at limiting the number of collisions experienced by elderly study participants during completion of an obstacle course.
Explains Dr. Mihailidis, “Our study shows that IWS has the potential to improve powered wheelchair safety and usability in older populations.”
This work was supported by the the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research CanWheel Emerging Team in Wheeled Mobility for Older Adults.
Evaluation of an intelligent wheelchair system for older adults with cognitive impairments. How TV, Wang RH, Mihailidis A. Journal of Neuroengineering and Rehabilitation. 2013 August. [Pubmed abstract]

A “phantom” is a cylinder with pumps and tubes to control the flow of simulated blood and material for X-Ray imaging.
How blood flows into and through tissue—blood perfusion—is an important characteristic of tumours, providing clues about the response to therapy. It can be measured non-invasively with dynamic contrast-enhanced (DCE) computed tomography (CT) scans, however observed changes in perfusion could be due to a number of factors such as a change in the tumour’s blood supply itself, or a change in the patient’s heart function. Techna Affiliated Faculty Dr. Catherine Coolens has developed a device—known as a “phantom”— that can be used to mimic blood flow in a tumour to calibrate and validate DCE-CT systems, a needed step before the images can be used as a quantitative clinical standard.
In a recent paper Dr. Coolens investigated methods of mathematically modeling blood flow, using measurements from this phantom system to test the predictions of the computer model. These models will help researchers better understand how blood flow carries drugs and nutrients into a tumour, which will lead to improved DCE-CT imaging as well as new chemotherapy treatment designs. Importantly, these simulations will help bridge the gap between what can be seen in a CT image and the underlying tumour physiology.
This work was supported by the Natural Sciences and Engineering Research Council of Canada.
Peladeau-Pigeon M, Coolens C. Computational fluid dynamics modelling of perfusion measurements in dynamic contrast-enhanced computed tomography: development, validation and clinical applications. Physics in Medicine and Biology. 2013 August 14. [Pubmed abstract]
Ankylosing spondylitis (AS) is a chronic disease characterized by inflammation of the joints bridging the spine and pelvis, progressing to the spinal vertebrae, leading to spinal fusion in severe cases. It affects predominantly young men, and it might take 5-10 years to make a diagnosis of AS. TWRI Senior Scientists Drs. Florence Tsui and Robert Inman recently examined the role of proteins which normally prevent excessive bone formation, Noggin (NOG) and Sclerostin (SOST), in AS.
Unlike other forms of arthritis such as rheumatoid arthritis, autoantibodies directed an individual’s own protein were not detected in AS for decades. The TWRI team discovered novel autoantibodies complexed with NOG and SOST in healthy individuals. Higher levels of these antibody complexes were found in AS patients and likely contribute to new bone formation at the site of inflammation in these patients leading to eventual bone fusion in the spinal joints.
“These findings suggest a possible link between autoimmunity and joint fusion in AS and provide the first evidence that AS is an autoimmune disease. More importantly, they hold the promise of earlier diagnosis and better management of this devastating disease.” says Dr. Tsui.
This work was supported by the Arthritis Society, the Canadian Institutes of Health Research (CIHR) and the CIHR Institute of Musculoskeletal Health and Arthritis.
Serum levels of novel noggin and sclerostin-immune complexes are elevated in ankylosing spondylitis. Tsui FW, Tsui HW, Las Heras F, Pritzker KP, Inman RD. Annals of the Rheumatic Diseases. 2013 July 26. [Pubmed abstract]
 TWRI Senior Scientist Dr.
TWRI Senior Scientist Dr.  TWRI Senior Scientist Dr.
TWRI Senior Scientist Dr.