
The hallmark motor symptoms of Parkinson disease (PD)—such as involuntary movements (called dyskinesia), rigidity, and tremors—are the result of a progressive loss of dopamine-producing neurons in a part of the brain called the basal ganglia. For most patients with PD, managing these symptoms requires long-term treatment with levodopa—a drug that replaces dopamine in the brain—or surgical options like deep-brain stimulation (DBS).
Unfortunately, these treatment options present numerous challenges. As the disease progresses, and more dopamine-producing neurons are lost, existing treatments become increasingly less effective at managing motor symptoms. Even in earlier stages, symptom control remains imperfect, with many patients experiencing periods known as “off” times—when motor symptoms return despite medication.
A recent Phase 1 clinical trial is laying the foundation for a revolutionary new treatment option—bemdaneprocel, an injectable treatment made from stem cells. These cells develop into dopamine-producing neurons, once inside the brain. Unlike current treatments, bemdaneprocel aims to target the root cause of PD motor symptoms by replacing lost neurons. The team, which included researchers from UHN’s Krembil Research Institute (Krembil)—Drs. Alfonso Fasano, Andres Lozano and Suneil Kalia—studied whether injecting bemdaneprocel into the basal ganglia of PD patients is safe.
The trial results were promising. The treatment was well tolerated, with no off-target effects such as graft-induced dyskinesias (new dyskinesias brought on by the injected cells) observed during the 18-month follow-up period. The trial also showed that the newly developed dopamine-producing neurons remained alive 18 months post-injection. Even more importantly, the team saw clinically significant improvements in participants’ motor symptoms—including reduced “off” times. The improvements were also found to be dose-dependent, as patients in the high-dose cohort showed greater improvements than those in the low-dose cohort. Although it is unclear whether these improvements were directly caused by bemdaneprocel treatment—as participants continued taking levodopa throughout the course of this trial—these initial findings offer hope for PD patients.
Despite its small size and focus on safety, the trial’s results suggest real potential for bemdaneprocel as a future treatment. The most recent post-study follow-up, at 24-months after treatment, adds further support. Larger, longer trials will help confirm the therapy’s safety and determine if these early improvements can be replicated. This early success brings us closer to a future where people with Parkinson have access to therapies that restore function, not just manage symptoms.
The first author on this study is Dr. Viviane Tabar, a Neurosurgeon at the Memorial Sloan Kettering Cancer Centre.
The senior authors on this study are Drs. Lorenz Studer and Claire Henchcliffe. Dr. Studer is the Director of the Center for Stem Cell Biology at the Memorial Sloan Kettering Cancer Centre. Dr. Henchcliffe is the Chair and Stanley van den Noort Professor of Neurology at the University of California Irvine School of Medicine.
Dr. Andres Lozano is the lead investigator on this study at UHN. He is a Senior Scientist at Krembil, and a Professor in the Department of Surgery in the Temerty Faculty of Medicine at the University of Toronto. Drs. Alfonso Fasano and Suneil Kalia also contributed to this study as co-investigators. Dr. Fasano is a Clinician Investigator at the Krembil Brain Institute (Krembil) at UHN, an Affiliate Scientist at the KITE Research Institute (KITE) at UHN, and a Professor in the Department of Medicine at the University of Toronto. Dr. Kalia is a Senior Scientist with Krembil and KITE, and an Associate Professor in the Department of Surgery in the Temerty Faculty of Medicine at the University of Toronto.
This work was supported by BlueRock Therapeutics and UHN Foundation.
BlueRock Therapeutics was founded in 2016 at UHN by Drs. Gordon Keller and Michael Laflamme. In 2019, BlueRock Therapeutics was fully acquired by Bayer AG.
Drs. Tabar and Lozano are scientific advisors for BlueRock Therapeutics (BlueRock). Dr. Sarva is a former consultant for BlueRock. Drs. Irion, Tomishima, Abid and Stemple are employees of BlueRock. Dr. Fasano receives royalties from Springer Nature, the company that publishes the journal, Nature. Drs. Tabar, Lozano, Fasano, Yu, Studer, and Henchcliffe also receive research funding from BlueRock outside the current study.
For a full list of competing interests, see the publication.
Tabar V, Sarva H, Lozano AM, Fasano A, Kalia SK, Yu K, Brennan C, Ma Y, Peng S, Eidelberg D, Tomishima M, Irion S, Stemple W, Abid N, Lampron A, Studer L*, Henchcliffe C*. Phase I trial of hES-cell-derived dopaminergic neurons for Parkinson’s disease. Nature. 2025 Apr 16. doi: 10.1038/s41586-025-08845-y.
*Contributed equally as senior authors on the publication.
A new study published in Nature by Dr. Steven Chan’s team at the Princess Margaret Cancer Centre (PM) suggests that metformin, a drug used to treat diabetes, may help prevent the expansion of mutant blood stem cells (hematopoietic stem cells, HSCs) linked to clonal hematopoiesis (CH), a condition associated with several illnesses, including blood cancer.
HSCs are immature cells that can develop into different types of blood cells. In CH, these stem cells acquire mutations that cause the abnormal expansion of the mutated cells. CH is linked to an increased risk of blood cancers, heart disease, and age-related inflammatory conditions such as chronic liver and kidney disease. Although suppressing the expansion of mutant HSCs may help prevent these health issues, no approved treatments are currently available.
The most common mutation in CH affects a gene called DNMT3A, which produces a protein, DNA methyltransferase, that helps regulate gene expression through a process known as DNA methylation. A specific mutation in this gene, DNMT3AR882, is linked to a significantly higher risk of developing acute myeloid leukemia (AML), making it an important target for preventive intervention.
To better understand how this mutation works, the researchers used experimental models with an equivalent mutation to DNMT3AR882. They discovered that cells with this mutation have higher metabolic activity than normal (wild-type) cells, which gives them a competitive advantage.
Metformin, a commonly used diabetes medication, works by inhibiting a protein called Complex I, which is involved in metabolism and energy production. Due to its effect on cellular metabolism, the team tested whether metformin could reduce the competitive advantage of mutant cells.
Through lab-based models, the researchers found that treatment with metformin reduced this competitive advantage. Advanced sequencing techniques also showed that metformin increased DNA and histone methylation potentials in mutant cells, helping to counteract the effects of the mutation and modify the activity of metabolism-related genes.
"Observing these metabolic changes in mutant cells marks the first time this feature has been demonstrated in CH,” adds Dr. Mohsen Hosseini, first author of the study. “This study is also among the first to reveal the crucial interplay between metabolism and DNA modification in CH, highlighting that these interactions can induce reversible changes to cellular characteristics."
“These findings are significant because they suggest that metformin could be used as a preventative treatment for people with CH with DNMT3AR882 mutations,” says Dr. Steven Chan, Senior Scientist at PM and senior author of the study. “More research and clinical trials are needed to investigate this further.”

(L-R), Dr. Mohsen Hosseini, first author of the study; Dr. Steven Chan, corresponding author of the study.
The first author of this study is Dr. Mohsen Hosseini, a Scientific Associate at the Princess Margaret Cancer Centre.
The senior author of this study is Dr. Steven Chan, a Senior and Allan Slaight Scientist at the Princess Margaret Cancer Centre and an Associate Professor in the Department of Medical Biophysics at the University of Toronto.
The following UHN Principal Investigators are co-authors of the study: Dr. Federico Gaiti, Scientist at PM; Dr. Aaron Schimmer, Director of PM, Dr. Gary Bader, Affiliate Scientist at PM; Dr. John Dick, Senior Scientist at PM, and Dr. Stephanie Xie, Scientist at PM.
The following Investigators from other Toronto Institutions are co-authors of the study: Dr. Shraddha Pai, Principal Investigator at the Ontario Institute for Cancer Research; Dr. Grace Egan, Clinician-Scientist at the Hospital for Sick Children.
This work was supported by The Princess Margaret Cancer Foundation, the University of Toronto’s Medicine by Design, the Leukemia Research Foundation, the Canadian Institutes of Health Research, the Canadian Cancer Society, the Terry Fox Research Institute, and the Ontario Ministry of Health.
Dr. Steven Chan has received research funding from the Centre for Oncology and Immunology in Hong Kong, Celgene/BMS, AbbVie Pharmaceuticals, Agios Pharmaceuticals, and Servier Laboratories. For additional competing interests, see the manuscript.
Hosseini M, Voisin V, Chegini A, Varesi A, Cathelin S, Manikoth Ayyathan D, Liu ACH, Yang Y, Wang V, Maher A, Grignano E, Reisz JA, D’Alessandro A, Young K, Wu Y, Fiumara M, Ferrari S, Naldini L, Gaiti F, Pai S, Egan G, Schimmer AD, Bader GD, Dick JE, Xie SZ, Trowbridge JJ, Chan SM. Metformin reduces the competitive advantage of Dnmt3aR878H HSPCs. Nature. 2025 Apr 16. doi:10.1038/s41586-025-08871-w.
For over a decade, a team of researchers at UHN’s Princess Margaret Cancer Centre (PM) has been advancing a revolutionary approach to cancer treatment. Their innovative work with Porphysomes—light-activated nanoparticles—has now reached a pivotal milestone: Health Canada’s approval to begin clinical trials. This homegrown innovation, funded from its inception by the Terry Fox Research Institute (TFRI) with $14.8 million in support, along with significant contributions from The Princess Margaret Cancer Foundation (PMCF) through multiple initiatives, including the Innovation Accelerator Fund, represents a major step forward in personalized cancer care.
The story began with a bold idea from Dr. Gang Zheng, a Senior Scientist and the Associate Research Director at PM. He envisioned using nanotechnology to both detect and treat cancer with unparalleled precision. “Porphysomes were a game-changer from the moment we discovered them,” says Dr. Zheng. “Their unique ability to accumulate in tumours and respond to light makes them an incredibly powerful tool for imaging and therapy.”
With initial support from TFRI and PMCF, Dr. Zheng and his team explored the safety and efficacy of Porphysomes across different cancer types. The nanoparticles’ ability to make tumours ‘glow’ under imaging significantly improved surgical precision, while their potential for photodynamic therapy (PDT) opened new doors for non-invasive treatments. These findings, published in major scientific journals, laid the foundation for translating the research into clinical applications. Building on this success, the research team is actively exploring commercialization opportunities for Porphysome-based therapeutics, with the goal of accelerating the journey from discovery to patient care and ensuring this Canadian innovation makes a lasting impact.
As the research progressed, key milestones brought Porphysomes closer to patient care. The team successfully refined the technology, enhancing its drug delivery capabilities and therapeutic impact. In recent pre-clinical studies, the team found that PDT with Porphysomes can stimulate the body's immune response to fight cancer. This has the potential to stop the spread of cancer and destroy distant tumours. The team will also explore the use of radioactively labelled Porphysomes and low doses of radiation to target deep-seated tumours, expanding the potential for Porphysomes to effectively treat cancers in a minimally invasive way.
Dr. Jonathan Irish, a Senior Scientist and Surgeon at PM, highlights the critical role of early support. “We explored Porphysomes’ safety and effectiveness across various cancers in lab studies, utilizing these unique nanoparticles for imaging to guide surgery and for light-based treatments such as PDT, which involves administering and activating non-toxic photosensitizers within tissues to kill surrounding cells,” he explains.
“This research is incredibly fascinating. It's these eureka moments that drive us forward, and technology offers new directions and modalities we must explore,” says Dr. Brian Wilson, a Senior Scientist at PM and the initial Principal Investigator of the TFRI-funded Porphysomes project.
With continued investment from TFRI, the Ontario Institute for Cancer Research (OICR), and PMCF, the team advanced Porphysomes toward regulatory approval. Health Canada’s recent authorization, for a first-in-human trial that will assess PET imaging with radiolabeled Porphysomes for advanced ovarian cancer patients, is a testament to the strength of this pan-Canadian effort.
With clinical trials set to begin soon, patients will be recruited to assess the safety and efficacy of Porphysomes. Dr. Amit Oza, a Senior Scientist and the Head of the Division of Medical Oncology and Hematology at PM, underscores the significance of this transition. “Moving from preclinical research to patient trials is a critical step,” says Dr. Oza. “Porphysomes act like a Trojan horse, infiltrating tumours and activating treatment from within. We are entering a new era where precision-guided, minimally invasive cancer treatments could become a reality.”
Beyond these initial trials, the future looks bright. The team is already exploring ways to expand the technology’s applications to other types of cancer and combination therapies. The ultimate goal is to integrate Porphysomes into standard oncology practice, improving outcomes for patients worldwide.
This achievement is more than just a scientific milestone—it is a testament to Canada’s leadership in cancer innovation. “This is a fully homegrown discovery, developed, tested, and now moving to the clinic entirely within Canada and led by UHN,” says Dr. Brad Wouters, Executive Vice President of Science and Research at UHN. “It’s a shining example of what can be accomplished when researchers, clinicians, and funding partners come together as One Team to push the boundaries of what’s possible.”
The journey from bench to bedside is never easy, but with Health Canada’s approval, Porphysomes are poised to change the future of cancer treatment. Thanks to the unwavering support of TFRI, PMCF, and other key partners, this Canadian innovation is on the cusp of making a global impact—one illuminated tumour at a time.
Dr. Gang Zheng is a Senior Scientist and the Associate Research Director at the Princess Margaret Cancer Centre. He is a Canada Research Chair in Cancer Nanomedicine, Tier I and a Professor at the University of Toronto.
Dr. Jonathan Irish is a Senior Scientist at the Princess Margaret Cancer Centre, member of the Cancer Clinical Research Unit (CCRU), and the Kevin and Sandra Sullivan Chair in Surgical Oncology. He is a Professor and the Head of the Division of Head & Neck Oncology and Reconstructive Surgery at the University of Toronto.
Dr. Brian Wilson is a Senior Scientist at the Princess Margaret Cancer Centre and a Professor of Medical Biophysics at the University of Toronto.
Dr. Amit Oza is the Head of the Division of Medical Oncology and Hematology, the Medical Director of the Cancer Clinical Research Unit at the Princess Margaret Cancer Centre, and the Daniel E. Bergsagel Chair in Medical Oncology. He is also the Co-Director of the Drug Development Program, a Senior Scientist at PM, a Scientist at the Ontario Cancer Institute, and a Professor of Medicine at the University of Toronto.
This work was supported by The Princess Margaret Cancer Foundation, the Terry Fox Research Institute, the Lotte & John Hecht Memorial Foundation, The Canadian Institutes of Health Research, and the Ontario Institute for Cancer Research.
The recipients of the 2025 Gairdner Awards—Canada’s most prestigious medical awards—have been announced. Among the awardees is University Health Network (UHN)’s Dr. Daniel De Carvalho, Senior and Allan Slaight Scientist at the Princess Margaret Cancer Centre.
Dr. De Carvalho has been awarded the 2025 Peter Gilgan Canada Gairdner Momentum Award for his impactful contributions to cancer epigenetics. His research has transformed the understanding of how epigenetic changes drive cancer and has led to novel approaches for early cancer detection and treatment. By identifying unique DNA methylation signatures in cell-free DNA, he and his team have developed liquid biopsy techniques capable of detecting cancer through a simple blood test.
These innovative methods offer a non-invasive and highly sensitive alternative to traditional diagnostic tools, opening new avenues for early detection and personalized treatment. "Receiving the Gairdner Momentum Award is an incredible honour and a testament to the collaborative efforts of my team and colleagues," says Dr. De Carvalho. "Our goal is to continue advancing cancer detection methods to improve patient outcomes and ultimately save lives."
Beyond his research in early cancer detection, Dr. De Carvalho has also made significant contributions to understanding how epigenetic therapies can enhance immune responses against cancer. His studies have revealed that epigenetic drugs can reprogram cancer cells to make them more recognizable by the immune system, a process named viral mimicry, offering promising strategies for combination therapies in immuno-oncology.
His research has also uncovered key insights into the interplay between epigenetics and tumour evolution, shedding light on how cancers develop resistance to therapies. By exploring these mechanisms, he is working toward designing more effective treatment strategies that can anticipate and counteract resistance, ultimately improving long-term outcomes for patients.
His innovative work has not only influenced cancer diagnostics but has also had a profound impact on the broader field of cancer research. Through his leadership and scientific vision, Dr. De Carvalho is shaping the future of cancer detection and treatment.
In addition to his scientific achievements, Dr. De Carvalho is deeply committed to mentoring and inspiring the next generation of researchers, supporting students and trainees in advancing their careers in cancer science.
The Gairdner Momentum Award recognizes scientists who have made exceptional contributions to health research and are poised for continued impact. Dr. De Carvalho's achievements exemplify this distinction, highlighting his ongoing commitment to advancing cancer research and improving patient care.
To learn more about this year’s recipients, read the press release here.
To learn more about Dr. De Carvalho’s research journey and discoveries, read his Meet @PMResearch story here.
Dr. Daniel De Carvalho was on a plane, about to depart for a conference, when his phone rang—a call from the Gairdner Foundation. Three hours later when he landed, he answered the call to learn the surprising news: The Gairdner Foundation was awarding him the 2025 Peter Gilgan Canada Gairdner Momentum Award.
“I was so excited,” said Daniel of the award, which goes to mid-career investigators for exceptional scientific research contributions. “The most rewarding part of receiving this award is that it renews our energy and focus to keep translating our discoveries into new diagnostics and therapies. It’s incredibly meaningful to see the work starts to make a difference for our patients and society.”
Dr. De Carvalho, a global leader in cancer epigenetics, and his team opened up a new avenue for cancer therapy through their work. He discovered the role of repetitive elements in our genome, in regulating viral mimicry response in cancer cells and provided the rationale for many cancer treatments undergoing clinical trials worldwide. The award also recognizes Dr. De Carvalho’s game-changing development of a novel blood-based test for early cancer detection, classification, and therapy monitoring, poised to revolutionize cancer management.
In 2015, Daniel’s team revealed that by activating parts of our own DNA that have an ancient root acquired from a viral infection, cancer cells can be disguised as viruses, triggering an immune response to fight cancers. They named this phenomenon the “viral mimicry”response.
The human immune system is well-prepared to fight viruses, but it struggles to recognize cancer cells. The discovery of viral mimicry made it possible to design better therapies that fight cancer through an antiviral route.
The team found double-stranded RNAs (dsRNA) in colorectal cancer cells, following the treatment with an epigenetic drug. dsRNA is a molecular structure typically present in host cells infected by viruses and an intermediate for viral replication.
“We saw a very strong antiviral response, but there was no viral infection,” says Daniel. These dsRNA fragments triggered a series of reactions that caused the cells to produce and secrete interferons, an antiviral signal to activate the immune system and destroy cancer cells.
But where did they come from if there was no virus in the first place? The study discovered that those dsRNAs were made from repetitive elements in the human genome called endogenous retrovirus—remnants of ancient retroviral infections that have become permanently integrated into our DNA. Unlike active retroviruses such as HIV, endogenous retroviruses no longer cause infection; instead, they are inherited like regular genes and passed down through generations.
The epigenetic drug used to elicit the viral mimicry response removes methyl groups, a chemical mark, on DNA. By demethylating DNA in the cancer cells, the drug unmasked endogenous retrovirus sequences that have long been silenced and domesticated in the genome, inducing a high level of dsRNAs in the cancer cells and an antiviral response. With more studies followed in the field, this discovery fundamentally changed our understanding of the physiological role of repetitive elements in the human genome and their function in anti-cancer therapies.
The team’s landmark paper was published in Cell, it was selected as one of the top 10 notable advances of 2015 by Nature Medicine, and was highlighted in the New England Journal of Medicine’s ‘Clinical Implications of Basic Research’ section.
Since then, the team has been working on pharmacologically manipulating this mechanism, to induce a strong antiviral response, for a stronger, anti-tumour therapy. Their discoveries have led to a patent filing and joint drug discovery program with pharmaceutical companies to develop novel anti-cancer therapies.
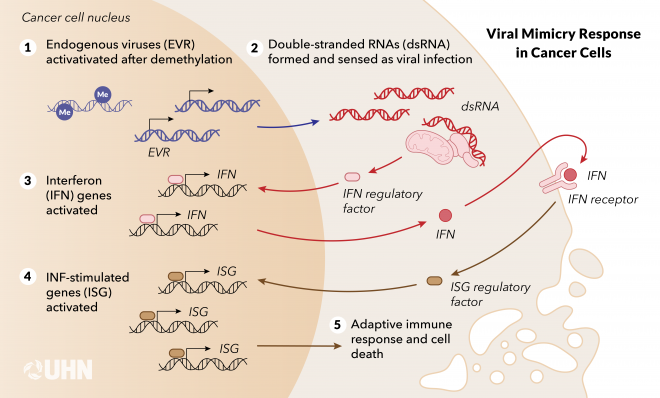
The viral mimicry state induced by epigenetic therapy (adapted from Cancer Discovery, 2021).
For Daniel, the discovery of viral mimicry response was a total surprise. At the time, scientists had observed the anti-cancer effects of some epigenetic drugs in clinical trials, but they didn't know why they worked.
"Our hypothesis was that the DNA demethylating drug activates tumour suppressor genes," Daniel recalls. "We absolutely were not expecting an antiviral response."
All the immune cells have the same genetic information, but they have very different functions. Different genes need to be activated or repressed in different cells to have a diversity of functions. This led me to the field of epigenetics, and later cancer epigenetics.”
Daniel earned his PhD study in Immunology at the University of São Paulo and completed a postdoctoral fellowship at the University of Southern California before moving to Toronto. He established his team at UHN’s Princess Margaret Cancer Centre and began a faculty position at the University of Toronto in 2012.
He feels a great sense of fulfilment and purpose in his research journey. “It's about enjoying the journey and process of doing science, not the destination. A lot of times, you can hit into dead ends, but sometimes you get to an interesting place. Surprise is always the strongest signal that you have found something new.”
It’s hard to treat cancer when a patient has an advanced disease. Another focus of Daniel’s group is to leverage epigenetic changes for early cancer detection, patient classification, cancer prevention, and interception.
“Compared to the normal human genome, cancer patients show massive changes in DNA methylation profiles. This creates opportunities to detect cancer early,” says Daniel.
His lab developed cfMeDIP-seq (cell-free Methylated DNA Immunoprecipitation and high-throughput sequencing), a highly sensitive method that isolates and sequences methylated DNA fragments in the blood. This method is revolutionizing the use of liquid biopsy, enabling a single blood sample to detect cancers in a cost-effective way. Unlike traditional methods, which require prior knowledge of a patient’s tumour-specific genetic mutations, cfMeDIP-seq identifies universal epigenetic changes shared across many cancers.
This next-gen approach was published in Nature in 2018, uncovering many methylated regions in plasma cfDNA from patients with different cancer types, even in early-stage disease. The method has shown robust cancer detection in seven different cancer types, including clinical evidence of detecting and classifying kidney cancers and intracranial tumours in two back-to-back publications in Nature Medicine.
Daniel, with Dr. Scott Bratman of PM and Dr. Anne-Renee Hartman, Maneesh Jain, and David Scheer, co-founded Adela, a biotech company, with the support of Commercialization at UHN and institutional investors. Their blood test was clinically validated and can detect head and neck cancer relapse up to 14.9 months earlier than the standard of care procedures, published in The Annals of Oncology.
Beyond detection, Daniel’s research has opened doors to cancer interception—stopping cancer before it fully forms. His viral mimicry study revealed that in premalignant lesions, cells that have yet to fully become cancerous can activate transposable elements, triggering a chronic immune response that eventually leads to immune exhaustion and tumour development. This work was published in Cancer Discovery and his team has explored using drugs to block this process, delaying or even preventing cancer formation in preclinical models.
"I'm really excited to deepen our understanding of how cancer initiates, which will lead to better therapies and interception strategies. In the field of liquid biopsy, I look forward to advancing the technology to make it more sensitive and specific, and to testing it in larger populations."
Meet PMResearch is a story series that features Princess Margaret researchers. It showcases the research of world-class scientists, as well as their passions and interests in career and life—from hobbies and avocations to career trajectories and life philosophies. The researchers that we select are relevant to advocacy/awareness initiatives or have recently received awards or published papers. We are also showcasing the diversity of our staff in keeping with UHN themes and priorities.
A recent study from the Krembil Brain Institute (Krembil) at UHN has revealed the essential role of the immune system in response to treatments for spinal cord injury (SCI). Comparing different laboratory models of SCI, a research team led by Krembil Senior Scientist Dr. Michael Fehlings discovered that cell therapy outcomes vary significantly between immunocompromised or immunodeficient models—which lack a fully functioning immune system—and immunocompetent models.
Cell therapies, such as human induced-pluripotent stem cell-derived neuron progenitor cell (hiPSC-NPC) transplants, are promising treatments for patients with SCI, many of whom have no other treatment options. However, to ensure cell survival and minimize rejection, preclinical models that scientists use to study the safety and feasibility of cell therapies, often exclude or downplay the immune system’s role. This trade-off affects how well these models replicate the real-world response to treatment.
Findings from this study suggest that this approach limits the relevance of these models to actual patients. Researchers observed that some immunodeficient lab models favour the development of neurons and oligodendrocytes from hiPSC-NPCs at the expense of the development of astrocytes, while astrocytes are the most common cell type that develop in immunocompetent models.
“Immunodeficient models seem to lack the intricate interplay between the transplant recipient’s immune system and the transplanted cells, resulting in an altered ratio of cells compared to what is expected in patients,” explains Dr. Fehlings. Further investigation also found that immunodeficient lab models show lower levels of cell death and stress along with higher levels of nervous system development and cell growth signalling.
Although it is not possible to replicate every aspect of SCI in preclinical models, this work highlights the need for more comprehensive models that better reflect the immune system’s role with transplant survival. Successfully addressing this challenge will bring cell therapies for SCI closer to clinical application than ever before.
The lead author of this study is Dr. Zijian Lou, a graduate research student at the Krembil Brain Institute in the Fehlings Lab.
The senior author of this study is Dr. Michael G. Fehlings, a Senior Scientist at the Krembil Brain Institute at UHN, the Head of the Spinal Program at UHN’s Toronto Western Hospital, and a Professor in the Department of Surgery at the University of Toronto.
This work was supported by Wings for Life, the Krembil Foundation, the Canadian Institutes of Health Research, and UHN Foundation.
Lou Z, Post A, Nagoshi N, Hong J, Hejrati N, Chio JCT, Khazaei M, Fehlings MG. Assessment of immune modulation strategies to enhance survival and integration of human neural progenitor cells in rodent models of spinal cord injury. Stem Cells Transl Med. 2025 Feb 11;14(2):szae090. doi: 10.1093/stcltm/szae090.
Researchers at Toronto General Hospital Research Institute (TGHRI) have found that a class of diabetes medications, including dapagliflozin—used with diet and exercise to lower blood sugar in adults with type 2 diabetes—is associated with reducing risk factors linked with cardiovascular disease and end-stage kidney disease (ESKD) in patients with type 1 diabetes (T1D).
People with T1D have an increased risk of cardiovascular disease and kidney failure. They are 10 to 30 times more likely to develop kidney failure compared to the general population. Additionally, a diagnosis of T1D before the age of 10 can lead to a threefold increase in the risk of heart disease. Thus, identifying therapies to help reduce these risks is essential.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors, a class of drugs that includes dapagliflozin, have been shown to lower these risks in people with type 2 diabetes and in people with kidney and cardiovascular disease associated with other conditions. However, no major studies have confirmed similar benefits for people with T1D.
To investigate this, the research team conducted a post hoc analysis of previous clinical trial data from the DEPICT-1 and DEPICT-2 studies. These trials were multicentre, phase 3, randomized studies that evaluated the safety and efficacy of dapagliflozin as an add-on therapy to insulin in lowering blood sugar levels.
The researchers used two risk prediction models to estimate changes in the 10-year cardiovascular disease risk and 5-year ESKD risk in 1,473 participants with T1D who took dapagliflozin.
After 24 weeks of treatment, participants taking dapagliflozin had an approximately 6% lower estimated risk of cardiovascular disease, as determined by one risk model, and a 9% lower risk, as determined by another, compared to those taking a placebo. Additionally, dapagliflozin lowered the risk of ESKD by approximately 8% compared to the placebo after 52 weeks of treatment. The benefits on kidney health were especially pronounced in participants with chronic kidney disease (CKD).
These findings suggest that SGLT2 inhibitors like dapagliflozin could play an important role in protecting the heart and kidneys in people with T1D. The authors have also been able to show similar potential benefits in separate models with another SGLT2 inhibitor called empagliflozin, which was used in the EASE clinical trials, and have published this work in the journal Diabetes Care. This study found that participants with T1D with the highest cardiovascular risk at the beginning of the trial may particularly benefit from these therapies.
Together, the study authors emphasize the need for dedicated clinical trials to confirm these benefits. As part of this effort, the team has initiated a new trial called “SUGARNSALT,” funded by the Canadian Institutes of Health Research, the Kidney Foundation of Canada, and Breakthrough-T1D, which will determine if there are long-term kidney benefits in this unique patient population. If proven effective, these medications could help individuals with T1D reduce their risk of life-threatening complications.
The first author of the study published in CJASN is Dr. Massimo Nardone, Postdoctoral Researcher at the Toronto General Hospital Research Institute.
The co-first authors of the study published in Diabetes Care are Luxcia Kugathasan, Ph.D. candidate at the University of Toronto and Toronto General Hospital Research Institute and Dr. Pritha Dutta, Postdoctoral Researcher at the University of Waterloo.
The senior author of both studies is Dr. David Cherney, Senior Scientist at the Toronto General Hospital Research Institute and Professor of Medicine at the University of Toronto.
This work was supported by UHN Foundation.
Nardone M, Kugathasan L, Sridhar VS, Dutta P, Campbell DJT, Layton AT, Perkins BA, Barbour S, Lam TKT, Levin A, Lovblom LE, Mucsi I, Rabasa-Lhoret R, Rac VE, Senior P, Sigal RJ, Stanimirovic A, Persson F, Stougaard EB, Doria A, Cherney DZI. Modeling Cardiorenal Protection with Sodium-Glucose Cotransporter 2 Inhibition in Type 1 Diabetes: An Analysis of DEPICT-1 and DEPICT-2. Clin J Am Soc Nephrol. 2025 Feb 7. doi: 10.2215/CJN.0000000641. Epub ahead of print.
Kugathasan L*, Dutta P*, Nardone M, Sridhar VS, Campbell DJT, Layton AT, Perkins BA, Barbour S, Lam TKT, Levin A, Lovblom LE, Mucsi I, Rabasa-Lhoret R, Rac VE, Senior P, Sigal RJ, Stanimirovic A, Persson F, Stougaard EB, Doria A, Cherney DZI. Modeling Cardiovascular Protection With SGLT Inhibition in Type 1 Diabetes: A Risk-Based Approach to Guide Therapy? Diabetes Care 2025. Mar 18. doi: 10.2337/dc24-2840. Epub ahead of print.
*Contributed equally
Research conducted at UHN's research institutes spans the full spectrum of diseases and disciplines, including cancer, cardiovascular sciences, transplantation, neural and sensory sciences, musculoskeletal health, rehabilitation sciences, and community and population health.
Learn more about our institutes by clicking below: